Biokar – Microbiology – Kits
Biokar Diagnostics is the microbiology division of the Solabia group, dedicated to the development, production and selling of culture media, supplements and detection kits for microbiology laboratories.
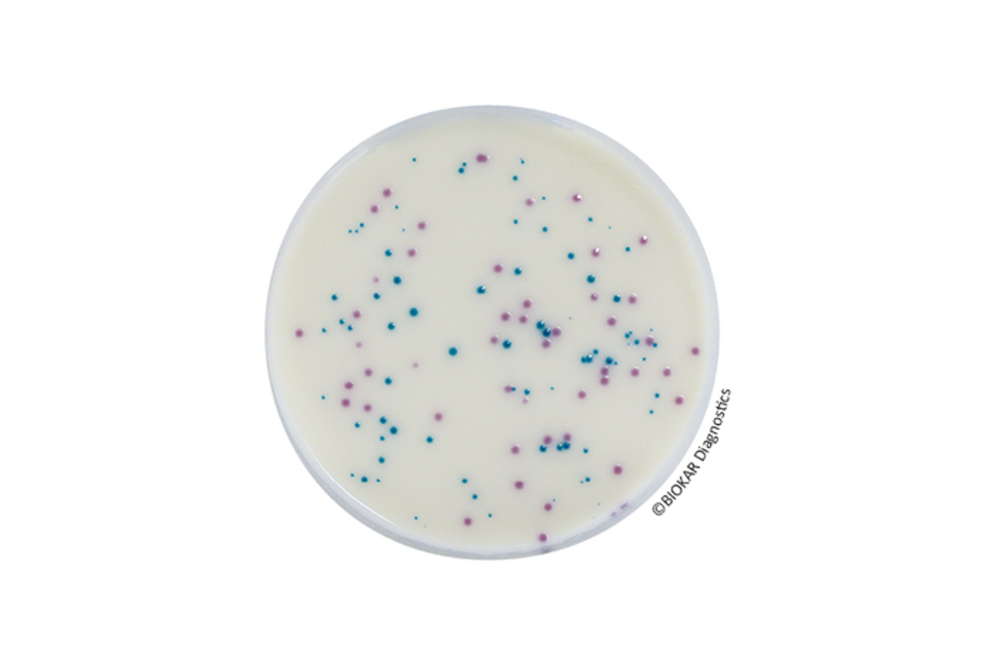
zBiokar Diagnostics offers specialized microbiology kits designed for the identification and enumeration of specific microorganisms in water samples.
Available Models
Culture Media Production by BIOKAR Diagnostics
BIOKAR Diagnostics is an industry leader with 50 years of experience in microbiology and we got a chance to visit their factory! Follow Julien Pastor from DKSH Technology and Athanassios Giannakopoulos from BIOKAR to visit this modern factory and learn their production of culture media, from peptones to ready-to-use media.
Key Features
- Standard Compliance: Kits are developed in accordance with international standards, ensuring reliable and recognized testing methods.
- Comprehensive Packaging: Each kit includes 25 microplates and 25 sterile, transparent adhesive covers, providing all necessary components for testing.
- Versatile Application: Suitable for analyzing various water types, including swimming and beach waters (both salt and fresh), surface waters, and post-treatment waters.
- High Specificity and Precision: Microtiter plate techniques offer enhanced specificity and accuracy compared to traditional methods, improving the reliability of results.
- Rapid Results: The design of these kits allows for quicker detection and enumeration of target microorganisms, streamlining the testing process.
Key Industries
- Agriculture
- Cosmetics & Personal Care
- Education & Academics
- Environmental
- Food & Beverage
- Medical
- Pharmaceutical
More Products
Biokar Diagnostics is the microbiology division of the Solabia group, dedicated to the development, production and selling of culture media,…